NOT ALL STEM CELLS ARE CREATED EQUAL
The goal to create an infinite supply of one's own stem cells came with its own challenges. Furthermore, we had to consistently achieve the same result for any person regardless of condition or age. For those who live and breathe cell culture, the obstacles outlined here are all too familiar. The field of stem cell medicine can only move forward if these issues are resolved.
At Hope Biosciences, we strive to create the highest quality cell product to ensure its therapeutic potential.

MORPHOLOGY
Challenge: Prolonged MSC culture often creates changes to morphology. Typically, the size of the cells becomes larger at further passages. The physical size increase comes with issues of overproduction of extracellular matrices causing cells to stick together. Giant, stuck-together cells are a pain to harvest and they pose safety issues, if administered.
Solution: Hope Biosciences proprietary cell culture method and media retains the original morphology of the MSCs. Please note: All therapeutics are delivered at Passage 4; however, we completed our process validation to include up to 12 passages. All testing conducted with cells from donors ranging from 30 to 80 years of age.

Unchanged morphology of HB-adMSCS from P0 to P12. Representative images of cell morphology at 70% confluence, 50X magnification for passages 0 through 12.
PROLIFERATION RATE
Challenge: Consistent cell growth rates are difficult to achieve and are the reason why autologous cell manufacturing is so difficult to standardize.
Solution: Our culture process maintains a linear proliferation rate.

The linear proliferation rate of HB-adMSCs from P0 to P12. Cumulative population doubling level (CPDL) means the proliferation and growth efficiency of HB-adMSCs which is calculated by CPDL =ln (Nf/Ni )/ ln2 with Nf is the final number of harvested cells and Ni is the initial number of seeded cells (Cristofalo, Allen, Pignolo, Martin, & Beck, 1998;Lee et al., 2016; Li, Zhang, & Qi,
DIFFERENTIATION CAPACITY
Challenge: When MSCs are taken outside of their normal environment and forced to grow in vitro, particularly in later passages, they have a tendency to differentiate. Keeping the cells in their undifferentiated state is critical to ensure their proper function when administered into the body.
Solution: Our proprietary cell culture process can maintain MSCs in their undifferentiated state in all passages while maintaining their differentiation capacity.
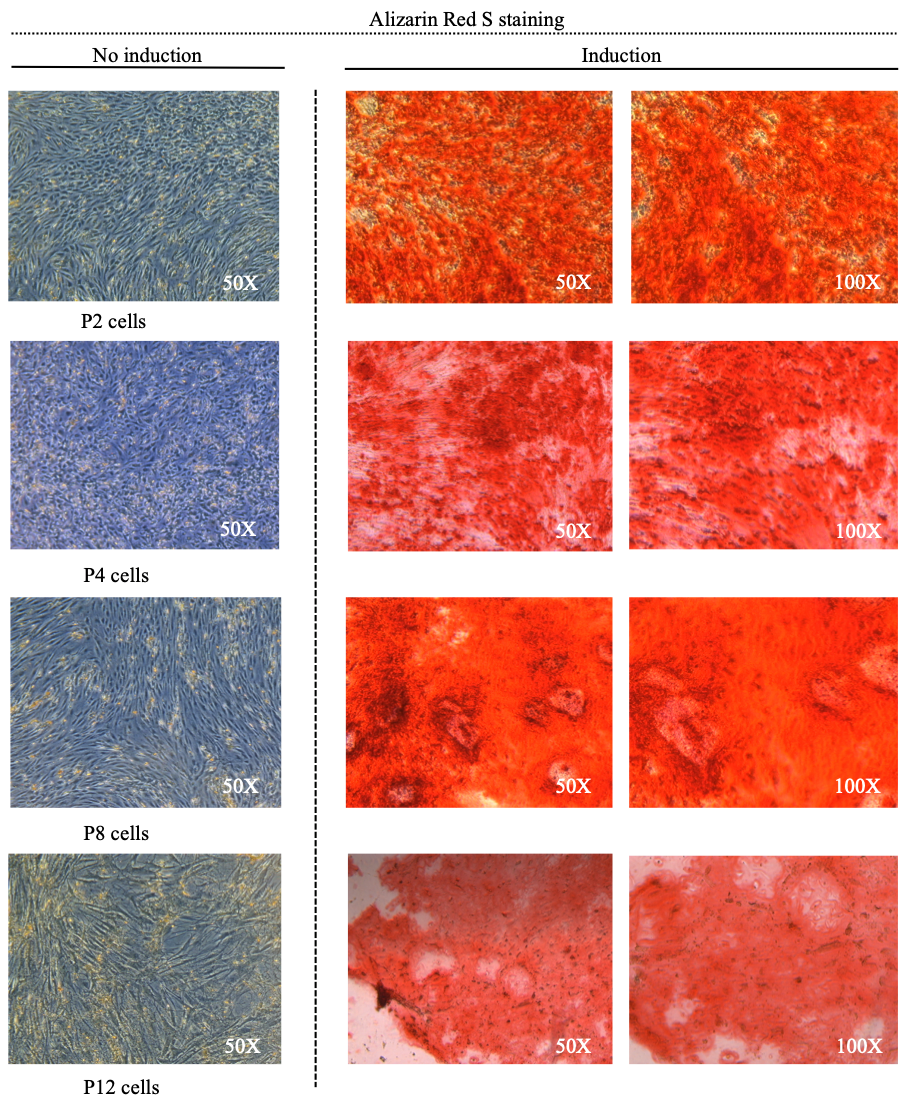
Osteogenic differentiation of HB-adMSCs(representative images).
Cells were stained with Alizarin Red S to determine calcium deposition. At day 21 post-osteogenic induction, high level of osteogenesis was characterized by the presence of strongly stained Alizarin Red S nodular structures with well-defined inter-nodular regions that not containing cells.
Chondrogenic differentiation of HB-adMSCs(representative images). Cells were stained with Toluidine Blue. At day 14 post-chondrogenic induction, chondrogenesis was characterized by the presence of strongly stained Toluidine Blue.

Adipogenic differentiation of HB-adMSCs (representative images). Cells were stained with Oil Red O. At day 14 post-adipogenic induction, MSCs contained large Oil Red O positive lipid droplets within their cytoplasm.
VIABILITY
Challenge: In the cell manufacturing process (culturing, thawing, cryopreserving, packaging, etc) any step can cause detrimental changes to the viability. Even the donor's age and condition can affect cell viability.
Solution: We always culture fresh, to order. Cells are never thawed and directly infused into the patient. Our standardized process takes cells from an individual's Master Cell Bank to manufacture the cell therapy. Upon release, cells can maintain an acceptable viability for up to 5 days (refrigerated).

Viability Validation 5 Donors of various age groups (40s, 50s, 60s, 70s and 80s) were tested for final product viability at 2-8 degrees Celcius. Represented data is the average viability of all 5 samples at the listed timepoints.
IDENTITY
Challenge: It's important to know that all the cells you receive for treatment are in fact stem cells. A mixture or heterogenous cell population may come with unknown risk. During cell culture, stem cells may begin to differentiate or mutate. Maintaining the same cell population becomes challenging through later passages.
Solution: As part of quality control, we require our cell therapy products to possess mesenchymal cell markers at high levels to ensure proper identity.

Histograms of HB-adMSCs’ cell surface markers across passages for sample HB170002-01. FACS analysis performed by University of Texas Health Science Center, Houston, Texas. Positive expression of CD73, CD90, CD29, and CD44
PURITY
Challenge: Population identity and purity go hand-in-hand. In later passages, we want to maintain the mesenchymal cell population while maintaining negative cell markers.
Solution: We can maintain high purity in our cell products.

Histograms of HB-adMSCs’ cell surface markers across passages for sample HB170002-01. FACS analysis performed by University of Texas Health Science Center, Houston, Texas. No expression of hematopoietic markers such as CD31, CD34, CD45, and HLA DR.
GENETIC STABILITY
Challenge: Stem cells can mutate during cell culture. Cells that have any genetic changes may be of risk to the recipient.
Solution: Safety is our first priority. We've gone through great lengths to show genetic integrity throughout the cell culture process. Karyotype and CGH analysis have been performed to show that genetic stability is maintained throughout the passages.
About Hope Biosciences
Hope Biosciences is a biopharmaceutical company developing adult stem cell therapeutics for a variety of clinical indications, and the only clinical grade adult stem cell banking facility in the nation serving both adults and newborns. Hope Biosciences occupies a unique position in the regenerative medicine space, noteworthy both for patented cell culture methods and effectiveness getting cells to patients through robust collaboration with academic and clinical research organizations. Hope Bio’s proprietary cell culturing process makes Hope Biosciences the gold standard in producing high volume, consistent, repeatable mesenchymal stem cells for clinical purposes, and Hope Biosciences actively partners with organizations and teams in need of cellular products for in vitro, preclinical, and clinical projects.
Learn more about Hope Biosciences at www.hope.bio.
About the Hope Biosciences Research Foundation
Hope Biosciences Research Foundation (HBRF) is on a mission to unlock the potential of regenerative medicine, bringing new hope to patients by dramatically accelerating research. A non-profit 501 (c)3 organization headquartered in Sugar Land, Texas (Houston area) and founded in 2020, we work primarily with chronic conditions, many of which our patients have been told are “incurable.” HBRF is closing gaps in research and care by not only facilitating healing for patients but developing sustainable solutions with the potential to right our currently crippled healthcare system.
Learn more about Hope Biosciences at www.hopebio.org.


Comentários